Slime Science
First, a disclaimer. Back in early August I wrote something about hoping to get back to a semi-regular blogging pattern, maybe once a week. Silly me. You would think I'd have learned when I say something like that, it is almost guaranteed that life will start to get really crazy, and blogging time will go from scarce to almost nonexistent. So, I wondered, could the opposite be true? If I tell you that I'm up to my neck with (delightful) teenagers, a seriously ill parent, and requests from offspring to take a look at fellowship essays, scholarship essays, nursing papers, and the like (seven of my nine needed editing advice this past week!), maybe, just maybe, in the next few weeks life will slow a tiny bit and I'll have time to take some of the ideas floating in my head and get them into words on the screen. Maybe???
Not having time to dig into more serious topics, I thought I'd share some fun science activities my youngest boys enjoyed this week. This year I get to teach chemistry on two levels - hurrah! Paul and Ben are dipping their toes into chemistry topics on an elementary school level, and Peter and Amanda are tackling the topic at a high school level.
I don't think the younger two have enjoyed anything more than playing with boron (as found in Borax) this week as they made slime and floam. Both of these recipes involve the same reaction, which uses glue and Borax to create polymer chains.
What's a polymer? Simply a large molecule made of repeated units. Naturally occuring polymers include shellac, natural rubber, and DNA. For synthetic polymers look no further than your tennis shoes, PVC pipes or Tupperware bowls.
Slime:
Solution A:
1/2 c. water
1/2 c. white glue
food coloring
Solution B:
2/3 c. warm water
2 t. Borax
1. Mix ingredients in Solution A together in small bowl.
2. In medium bowl, mix ingredients in Sol'n. B. The Borax won't fully dissolve, but stir thoroughly to dissolve as much as possible.
3. Slowly pour Solution A into Solution B. Don't stir!
4. Gently roll the Solution A around in the Borax solution 4-5 times.
5. Lift out the glob and gently knead it for a few minutes.
6. Enjoy! Store the slime in an airtight container, preferably in the fridge.
"Floam"
This one is just a variation of the above, but makes a cool moldable Floam-like substance. This recipe comes from the curriculum I'm using with Paul and Ben, R.E.A.L. Science Odyssey: Chemistry, which I think is pretty super. Currently we're making our way through the periodic table, family by family, meeting many of the elements and learning basic chemistry terms.
Solution A:
1/4 c. glue
1/4 c. water
Food coloring, if desired
Solution B:
1/2 c. water
2 t. Borax
You will also need: 1 c. styrofoam or polystyrene beads (I stole some from one of our bean bag chairs)
Mix Solution A and Solution B in separate bowls. (As in the first recipe.) Pour Solution A into a gallon zip bag, then add 2 Tablespoons of Solution B. Don't mix it yet. Quickly also add in the polystyrene beads. Squeeze out the air from the bag, tightly seal, then knead gently for several minutes. The putty will quickly change consistency. Take out of bag when it has solidified as much as it is going to. Now you can play with this concoction! Mold it, make bouncy balls, or just explore it's weird properties. If you leave a sculpture out, it will dry and you can paint it. If you store it in an airtight container, you can keep it for some time.
Here's what's happening:
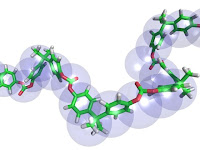
 The polyvinyl acetate in the glue reacts with the Borax (sodium borate) to form a flexible polymer The Borax acts as a crosslinking agent to connect the polyvinyl acetate molecules into long chains.
The polyvinyl acetate in the glue reacts with the Borax (sodium borate) to form a flexible polymer The Borax acts as a crosslinking agent to connect the polyvinyl acetate molecules into long chains.
Want to try even more recipes or check out other properties of slime? This link has some great ideas including a Metamucil Flubber and Silly Putty.
 |
I don't think the younger two have enjoyed anything more than playing with boron (as found in Borax) this week as they made slime and floam. Both of these recipes involve the same reaction, which uses glue and Borax to create polymer chains.
What's a polymer? Simply a large molecule made of repeated units. Naturally occuring polymers include shellac, natural rubber, and DNA. For synthetic polymers look no further than your tennis shoes, PVC pipes or Tupperware bowls.
 |
| Ben used a straw to make this slime balloon. |
Solution A:
1/2 c. water
1/2 c. white glue
food coloring
Solution B:
2/3 c. warm water
2 t. Borax
1. Mix ingredients in Solution A together in small bowl.
2. In medium bowl, mix ingredients in Sol'n. B. The Borax won't fully dissolve, but stir thoroughly to dissolve as much as possible.
3. Slowly pour Solution A into Solution B. Don't stir!
4. Gently roll the Solution A around in the Borax solution 4-5 times.
5. Lift out the glob and gently knead it for a few minutes.
6. Enjoy! Store the slime in an airtight container, preferably in the fridge.
"Floam"
This one is just a variation of the above, but makes a cool moldable Floam-like substance. This recipe comes from the curriculum I'm using with Paul and Ben, R.E.A.L. Science Odyssey: Chemistry, which I think is pretty super. Currently we're making our way through the periodic table, family by family, meeting many of the elements and learning basic chemistry terms.
Solution A:
1/4 c. glue
1/4 c. water
Food coloring, if desired
Solution B:
1/2 c. water
2 t. Borax
You will also need: 1 c. styrofoam or polystyrene beads (I stole some from one of our bean bag chairs)
Mix Solution A and Solution B in separate bowls. (As in the first recipe.) Pour Solution A into a gallon zip bag, then add 2 Tablespoons of Solution B. Don't mix it yet. Quickly also add in the polystyrene beads. Squeeze out the air from the bag, tightly seal, then knead gently for several minutes. The putty will quickly change consistency. Take out of bag when it has solidified as much as it is going to. Now you can play with this concoction! Mold it, make bouncy balls, or just explore it's weird properties. If you leave a sculpture out, it will dry and you can paint it. If you store it in an airtight container, you can keep it for some time.
Here's what's happening:
 The polyvinyl acetate in the glue reacts with the Borax (sodium borate) to form a flexible polymer The Borax acts as a crosslinking agent to connect the polyvinyl acetate molecules into long chains.
The polyvinyl acetate in the glue reacts with the Borax (sodium borate) to form a flexible polymer The Borax acts as a crosslinking agent to connect the polyvinyl acetate molecules into long chains. Want to try even more recipes or check out other properties of slime? This link has some great ideas including a Metamucil Flubber and Silly Putty.
 |
| Ben holds the Floam below and the slime above. |


Comments